Pythium species are soil-borne diseases that pose a substantial danger to agricultural productivity around the world and generate significant economic losses. Pythium spp. can be controlled with chemical fungicides and other established management techniques. The problem is that as people pay greater attention to environmental and human health concerns, alternative methods are urgently needed. It has already been tried utilizing natural extracts, adjusting planting conditions, and using plant growth-promoting rhizobacteria and screening plants for disease tolerance. There have been significant advancements in the field of Pythium disease control, including the use of chemical and physical treatments, biocontrol, and host plant defense.
What is Pythium Root Rot?
The initial signs of Pythium Root Rot are often circular lesions that range in size from 2 to 5 centimeters, although they can grow to a maximum of 15 centimeters in diameter. When the weather is hot and humid, the spots emerge out of nowhere. It’s possible for these spots to grow quickly.
When does Pythium Root Rot occur?
The best circumstances for Pythium are warm nights (above 20°C) and hot days (above 30°C) with rainy weather.
Bạn đang xem: How To Control Pythium? Comprehensive Guide
Following the administration of nitrogen, a plant’s lush development is especially vulnerable to assault. Pythium is a water-borne illness that thrives in water-logged soil and is more widespread at higher temperatures.
Regardless of the soil type, the disease can take hold if the correct conditions are available. The additional watering that occurs during seeding creates the ideal conditions for this phenomenon to occur.
What damage or effect will Pythium Root Rot have?
Cold-season grasses are the most vulnerable to Pythium spp. infections, but all turfgrass species are susceptible. There are a number of Pythium species that are known to cause turfgrass injury.
Crater-like depressions are left on the turf after the sick spots dry out, preventing play.
Also known as crown and root symptoms, Pythium spp. is known to produce an overall loss in the vigor of the turf. Particularly on cool-season, intensively managed turf, this decline can affect only a few spots or a wide area.
All turf grass species are susceptible to Pythium spp., a fungus that mostly affects golf and bowling greens. Preventative treatments like Signature Stressgard or curative treatments like Banol are recommended by Bayer.
Physical and chemical control
Species of Pythium are found in abundance in both soil and water (Senda et al., 2009; Toda et al., 2015; Uzuhashi et al., 2015). Because of their adaptability, they may thrive in a wide range of temperatures, with the optimal range varying from as low as 13°C to as high as 35°C depending on the species There was a strong correlation between nutrient solution temperature and plant root necrosis produced by P. myriotylum in hydroponic culture (Fortnum et al., 2000).
Other studies have shown that a low temperature can reduce the amount of colonized and browned roots (Sutton et al., 2006). It’s worth noting that temperature affects Pythium sppaggressiveness .’s as well as their fungicide susceptibility. Torulosum and P. torulosum showed more aggression at 18°C and 23°C, respectively, while P. sylvaticum showed greater aggression at 13°C, as reported by Matthiesen et al. Fungicide sensitivity increased with temperature for P. oopapillum and P. torulosum, interestingly.
In addition to the temperature, the amount of water in the body plays an important role in determining the intensity of symptoms. Partial saturation, according to Gent and McAvoy (2011), decreased biomass without affecting flower development or the content of plant nutrients. Root rot symptoms were reduced in three out of five experiments, and even Pythium on agar recovered at a lower rate than standard saturation in several experimental groups (Elmer et al., 2012). The biomass and stem height were lowered by 10-20% by rapidly irrigating and draining to simulate partial saturation, but no symptoms appeared under partial saturation (Gent et al., 2011).
Petunia hybrida ‘Dreams Red’ roots were less likely to be infected by P. aphanidermatum when water content was kept under tight control (0.2 m3/m3) as opposed to those of plants treated with 0.4 m3/m3 and cyclic water (0.18 to 0.43 m3/m3). Cycle therapy does have a lower fatality rate (Wheeler et al., 2017). Pythium root rot can be prevented by altering water properties including oxidation-reduction potential (ORP) as well as managing water content. Using chlorinated water, for example, wiped out Pythium zoospore within minutes (Lang et al., 2008).
Hydroponically infected tomatoes, on the other hand, remained healthy due to the high oxygen concentration in solution (Chérif et al., 1997). Pythium can also be controlled with the use of a filter and UV light. Although UV light failed to cure symptoms and reduced yield even if it could limit the zoospore population, filtering only delayed root rot transmission for one or two weeks depending on the pore diameter (Goldberg et al., 1992; Zhang and Tu, 2000).

Metalaxyl, azoxystrobin, fosetyl-Al, pyraclostrobin, and trifloxystrobin are just some of the commercial chemical fungicides that are effective against Pythium spp. (Taylor et al., 2002; Stiles et al., 2005; Lookabaugh et al., 2015; Matthiesen et al., 2015). Fungicides, on the other hand, shown varying levels of efficacy across a range of plants, including Pythium spp. In some fungicides, the EC50 value might rise by more than 100 times as a result of environmental temperature (Matthiesen et al., 2015).
Xem thêm : How To Grow Gypsophila? Comprehensive Guide
Rather than using chemical fungicides, some people prefer to use chemical agents that can boost the defenses of plants. Pre-treatment of turmeric (Curcuma longa L.) with Acibenzolar-S-methyl, a functional counterpart of salicylic acid, increases the activity of peroxidases and protease inhibitors, resulting in decreased cell death following P. aphanidermatum inoculation, for instance (Radhakrishnan et al., 2011). fungicides, the silver ion dissolved in silver-coated cloth by Zhao et al. (2000) effectively reduced root rot symptoms. Under hydroponic circumstances, nonionic surfactants were surprisingly effective at controlling the spread of Pythium zoospores (Stanghellini et al., 1996).
P. abappressorium was suppressed by Brassica juncea seed meal for at least 12 weeks on apple seedlings in addition to the use of chemical agents (Weerakoon et al., 2012). Poinsettia (Euphorbia pulcherrima) illness symptoms were greatly reduced under partial saturation ebb and flow systems following inoculation with P. aphanidermatum, according to Gent Gent and Elmer (2017). Aqueous polymer sodium silicate solution inhibited Pythium in planta, but not in vitro (Mohsen et al., 2015). Using natural extracts like Vitex agnus-castus extract methanolic extract, researchers found that it not only had overall antifungal activity against P. ultimum, but it also activated some pathogenesis-related proteins in tomato seedlings, which were found to be completely safe.
Biocontrol
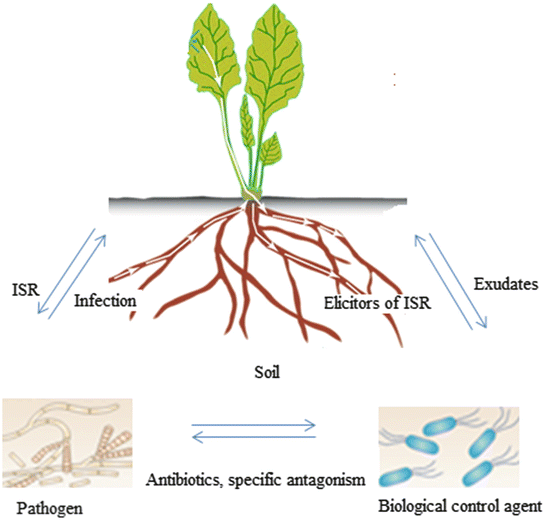
Chemical fungicides aren’t the only options available for disease management; biocontrol is also viewed as a viable alternative. Many biocontrol agents have been discovered by utilizing the competition between microorganisms. Preemergence damping-off and Pythium colonization on cotton seed were efficiently controlled by Enterobacter cloacae and Erwinia herbicola, which were as effective as fungicides at 15°C (Nelson, 1988). All 11 isolated endophytic actinobacteria from various plants, according to Misk and Franco (2011), demonstrated antibacterial potential against P. irregulare. Pythium damping-off control was also shown in strains isolated from irrigation wells (Tabli et al., 2018). A list of Pythium spp. biocontrol agents can be found in Table 1.
A few biocontrol drugs can even stimulate plant growth while alleviating symptoms at the same time. (Suwannarach et al., 2015). Rhizoctonia solani AG-8’s illness symptoms were reduced by six strains of Pseudomonas spp., which were the appropriate biocontrol bacteria (Mavrodi et al., 2012). It has also been shown that two strains of them boosted wheat seedling shoot and root length and weight, while suppressing pathogenic bacteria (Mavrodi et al., 2012).
A study published in 2015 by Kipngeno et al. found that the dry mass of tomato seedlings was dramatically increased when the seeds were coated with Bacillus subtilis and Trichoderma asperellum. There were 10.87 percent and 15.3 percent post-emergence damping-off proportions as compared to the control (63.9 percent ). Biocontrol agents, on the other hand, are able to alleviate symptoms by generating plant defenses. Biocontrol agent inoculation increased endogenous salicylic acid buildup in cucumber roots, for example, by Pseudomonas corrugata strain 13 and Pseudomonas aureofaciens strain 63-28. (Chen et al., 1999).
These two plant growth-promoting rhizobacteria, in addition to P. aphanidermatum, also boosted the activities of peroxidase (PO) and polyphenol oxidase (PPO) when applied to cucumber root rot induced by this pathogen (Chen et al., 2000). As a result of inoculation with two nonpathogenic Fusarium strains and subsequent increase in benzyl isothiocyanate and glucotropaeolin, the host plant’s resistance to P. ultimum was increased (Ishimoto et al., 2004). A mycoparasite, Verticillium lecanii (Zimm. ), was reported by Benhamou and Brodeur (2001) to hinder the colonization of P. ultimum and trigger host plant defenses to limit pathogen penetration in the epidermis and outer cortex by microscopy.
The key to effective biocontrol is maintaining the population of the biocontrol agent. Upon inoculation of the sweet pepper with the biocontrol agents P. chlororaphis Tx-1 and P. aphanidermatum, the Tx-1 biocontrol agent was observed to maintain a steady density, but rapidly dropped in the non-inoculated root (Chatterton et al., 2004). Using chitosan, Postma and colleagues (2009) found that Lysobacter enzymogenes 3.1T8’s ability to control the pathogen P. aphanidermatum in cucumber was boosted and the bacterial population grew.
Aside from the direct use of microbes, compost can also be used to control Pythium. Pythium wilt disease on cucumber plants had its presence inhibited by commercial compost, which contained (0.056-0.36 percent) fungal Cystobasidiomycetes and (0.011-0.018 percent) Acidobacteria Gp14 (Yu et al., 2015). andosols and ferralsols in Cameroon were found to have a lower illness index when organic matter content and sand and clay content were compared (Adiobo et al., 2007). By pasteurizing and treating with fungicide and bactericide, andosol suppression was dramatically reduced. A higher fungal population in recolonized and used rockwool caused a considerable rise in disease incidence as compared to using autoclaved rockwool (Postma et al., 2000). Pythium growth was shown to be highest in sterilized soil, and lowest in sterilized soil containing compost, suggesting that soil microflora can restrict Pythium’s ability to thrive.
There are a number of biological agents that can be used to control Pythium species.
Host plant defense
Following infection, root necrosis can be effectively prevented by activating defense signaling pathways like JA signaling, mitogen-activated protein kinase (MAPK) signaling, and wall-associated kinases (WAKs) (Zhu et al., 2019). Salicylic acid and gibberellic acid signal pathways have been found to be crucial in rice (Oryza sativa) defense against P. graminicola, while Arabidopsis protection against P. irregulare has been linked to JA, abscisic acid, and the ethylene signal pathway.
It was revealed that apart for a crucial signal pathway, the expression of pathogenesis-related-10 gene in moss tissue boosted resistance to Prion irregulare. Resistance to P. irregulare was attributed to SWEET2 activity in another study, which reduced sugar availability in the rhizosphere (Chen et al., 2015). Zingiber zerumbet L. Smith, a wild ginger cousin, was recently discovered to have a ZzR1 Pythium resistance gene.
In addition to studying plants’ molecular makeup, several scientists also work on breeding and screening projects. The ‘KS93U161’, ‘OH708’, and ‘Sunco’ genotypes were shown to be the most resistant to Pythium root rot based on the number of root tips and the total root length (Higginbotham et al., 2004). Of the 19 varieties tested for Pythium root rot resistance in the Caladium genus, 4 were somewhat resistant (Deng et al., 2005).
Furthermore, Nouri-Ellouz et al. (2006) obtained certain hybrid lines that demonstrated better resistance ability against P. aphanidermatum by undergoing somatic hybridization with potato ‘Aminca-Cardinal’ and ‘Cardinal-Nicola.’ One line was even reported as 100% resistance. Phosphoric compounds were found as active components in all plants that showed resistance to Pythium. According to Temgo and Boyomo (2002), the cocoyam clone’s resistance ability was measured by the concentration of antifungal phenolic chemicals. Rosmarinus multiflora ‘Matsushima No. 3’ showed excellent resistance to P. helicoides, according to Li (2006) (Table 2). Additional research conducted by Zhuang et al. (2012) found that R. ‘Matsushima No. 3’ phenolic compounds suppressed P. helicoides in vitro germ tube growth (Figure 1). Figure 2 depicts the disease symptoms of resistant and susceptible cultivars.
After P. helicoides B-5 inoculation, roses had lower root rot severity and a higher infection rate. To put it another way:
Xem thêm : How To Store Turmeric Rhizome? Comprehensive Guide
a Li et al. described the computation methods (2007).
Percentage of plants infected by a disease is determined by dividing the total number of plants infected by the total number of plants.
R. ‘Matsushima No. 3’ and R ‘Nakashima 91, which include bound phenolic compounds, have been shown to have protective effects on germ tube elongation. This version is based on Zhuang et al. (2012).
x) Fisher’s LSD test (n=3) finds significance at p=0.05 for values with distinct superscripts.
y) The root extracts of 5 g (fresh weight) root mL-1 contained bound phenolic compounds (the original liquid).
A 5 mm inoculum of P.helicoides B-5 was placed in the center of a petri dish and was incubated for 12 hours with phenolic compounds (or 99 percent ethyl alcohol). The germ tube elongation area was then measured every 12 hours.
R. multiflora ‘Matsushima No.3’ and R ‘Nakashima No.91’ roots following inoculation with P. helicoides and noninoculation, respectively, one week later.
These days, the use of external stimulation to activate the plant’s defenses is a trendy topic. It is possible to activate the plant’s defense mechanisms using a variety of compounds. Silica can boost cucumber resistance to P. ultimum by producing electron-dense layers along primary and secondary cell walls as well as pit membranes of xylem vessels and dramatically increasing the percentage of cells filled with phenolic-like material, as an example. P. aphanidermatum inoculation reduced cell death when salicylic acid was pre-treated with peroxidases and protease inhibitors, on the other hand (Radhakrishnan and Balasubramanian, 2009).
Host plant defense is commonly utilized around the world in the management of soil-borne disease through grafting. Cucumbers grafted onto cucurbit hybrid rootstock ‘Titan’ and ‘Hercules’ showed no symptoms and grew and produced more fruit than cucumbers grown on their own (Al-Mawaali et al., 2012).
Symptoms and Signs
The root tip and newly germinated seedlings are particularly vulnerable to Pythium. The fungus can cause quick brown to black rot of the primary root and even travel up into the stem tissue once it has gained access to the root. A new root system may be formed as the soil dries, allowing the plant to recover or completely avoid the illness. More roots are killed and the plant may wilt, stop growing, or even collapse and die in damp conditions caused by poor soil drainage or excessive irrigation. Bulbs of sensitive plants turn brown to black, dry out, and harden into a mummy-like structure.
Comments on the Disease
Pythium root rot, commonly known as water molds, is caused by pathogens that may be found in almost all farmed soils and attack plant roots when they are wet. Contamination of potting mixes in containers is possible as a result of the introduction of these fungi into the environment by fungus gnats and beach flies. Many Pythium species exist, some of which are beneficial in the sense that they compete with or parasitize pathogenic species. Pythium ultimum, one of the numerous pathogenic species, has a very broad variety of host ranges, whereas other pathogenic species have more restricted host ranges.
P. aphanidermatum, for example, is only a pathogen at high temperatures (over 77°F), while others are only active at low soil temperatures. Infected soil has a moisture content of 70% or more of its potential water capacity. Pathogenic Pythium species are likely to be present in the soil from a field.
Spores of some Pythium species differ from those of other species. In water, sporangia-produced zoospores are able to move. They may survive lengthy periods of drying because oospores have been dormant for a long amount of time after the sexual process. Chlamydospores, which are asexual and have strong cell walls, are also produced by some species. These buildings can be used as shelters for the winter or as a means of survival. In general, sporangia and zoospores do not survive for long periods of time in air or dry soil.
Conclusion
Pythium spp. are responsible for a great deal of damage to the global economy. As our knowledge of Pythium has grown, we’ve been able to develop more realistic approaches to treating the condition. For the most part, chemical fungicides have been the most extensively employed method since they are simple to use and effective. Aside from the use of fungicides and other measures to control water content, regulating temperature, and using compost to reduce the risk of Pythium infection in crops, these methods can only delay the disease’s development because they damage Pythium’s ideal growing environment.
A large number of biocontrol agents have been found and studied in recent decades as a developing topic. Plant growth-promoting rhizobacteria have recently received more attention than hostile microbes. Biocontrol in the lab appears to be a huge success thus far. Unfortunately, the field test’s efficiency pales in comparison to that of the lab test. Maintaining a stable population of biocontrol agents and mitigating potential threats to other plants and microbes are still issues that must be addressed. These past few years have seen a flurry of research into how plants react to Pythium. Disease resistance has been found to have a role in a number of essential plant signaling pathways.
Many plants were discovered to be tolerant, although total resistance has not yet been documented. A linear relationship between average pod rot and sampling units with pod rot at low disease incidences in peanut plantations was found, despite the fact that disease management was employed by Wheeler et al. (2016), and they believed that this research would help farmers manage the field more scientifically. Pythium control will almost certainly necessitate the use of more than one approach. Biocontrol agents and fungicides with lower toxicity and greater efficacy will be discovered as the research
Nguồn: https://iatsabbioneta.org
Danh mục: Guide










