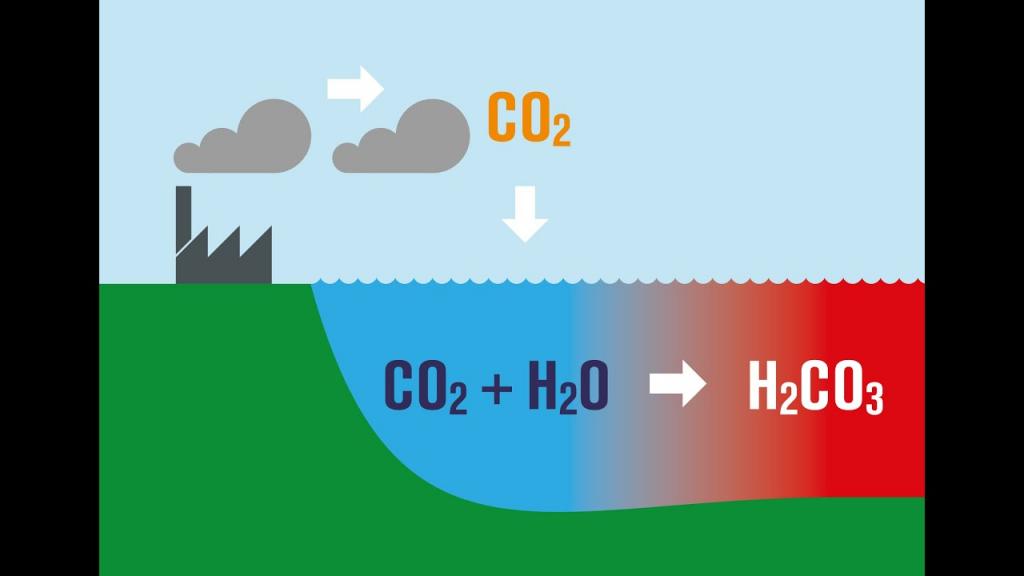
pH is a measure of water’s acidity or alkalinity. The pH scale has a range of 0-14, with a value of 7 indicating neutrality. Drinking water typically has a pH level between 6 and 8.5. Geological conditions and types of minerals found in local rock play an important role in determining pH at a given location. Acid rain can also have an effect on the pH of the soil. Acidic water is that which has a pH less than 7 and is therefore potentially corrosive. Metals can be leached from plumbing systems by acidic water (low pH). This can cause pipes to burst. Lead from lead pipes or copper from copper pipes might create health issues if they leach out of the pipes. The taste of water can be affected by the alkalinity of water with a value greater than 7. Drinking water with an alkaline pH can have a “soda” flavor. Corrosion can also be a concern with plumbing. There are three main types of pH adjusting devices, and we’ll go over each one in turn.
- 10 Disease Fighting Foods To Eat Right Now. Helpful Information!
- How Does Healthy Eating Prevent Diseases? A Must Read!
- How To Bring Blood Pressure Down Naturally? Complete Step-by-Step Guide
- Feet Hurt In The Morning When I Get Out Of Bed: Reasons Why!
- Why Muscle Recovery Gets Harder With Age? 6 Ways to Support Muscle Recovery as You Get Older
Neutralizing filters
How neutralizing filters work
If the water is acidic, a neutralizing filter is needed (low pH). The pH of the water is raised by adding a neutralizing agent to this simple treatment equipment. Water hardness may rise as a result of the neutralization process, though.
Bạn đang xem: How To Acidify Water? A Few Tips to Remember
Point-of-entry devices known as neutralizing filters adjust the pH of water to a neutral level (about 7), reducing or eliminating corrosion problems in plumbing. Water with a pH of more than 6 can be treated with calcium carbonate, whereas water with a pH of less than 6 can be treated with synthetic magnesium oxide.
Filtered water passes through a calcium carbonate or synthetic magnesium oxide medium-filled filter. The water’s pH level rises as a result of the substance dissolving in it.
Capacity
This procedure takes time and the flow rate should not exceed 3 gallons per minute per square foot of filter bed area in general. Shallower beds will not give enough neutralizing time if they are any shallower than 32 to 36 inches in depth.
Maintenance
It’s essential to maintain all treatment systems on a regular basis. A neutralizing filter’s substance must be replenished and the filter backwashed on a regular basis.
The neutralizing filter’s lifespan can be extended by removing solid particles from the water with a cartridge filter before installing the neutralizing filter.
Special considerations
If calcium and magnesium are utilized in the neutralizing filter, they may increase or induce water hardness. The neutralizing filter should be followed with a water softener if hard water becomes a problem. People on a low-sodium diet may not be able to drink water that has been treated with sodium.
Because water must pass through finely ground neutralizing material, neutralizing filters can cause water pressure loss in addition to water hardness.
No corrosion protection is provided for the pressure tank or well pump when neutralizing filters are used. If the flow rate is large, a liquid injection system (see below) may be preferable to a neutralizing filter since it is installed before to the pressure tank and so protects the tank and the plumbing system from corrosion.
Soda ash/sodium hydroxide injection
How soda ash/sodium hydroxide injection works
If the water is acidic, this treatment process is used (low pH). By adding sodium carbonate or hydroxide to the water, the pH of the solution is raised to a near-neutral range. They don’t produce hardness issues in treated water like neutralizing filters do.
A point of entrance is an injection system. A corrosion-resistant chemical feed pump raises the pH of the water by injecting soda ash or sodium hydroxide solution. Well casing and pump should be protected from corrosion by adding the solution straight into the well.
If disinfection and neutralization of the water are required, a chlorine solution (sodium hypochlorite) can be added to the injection system along with the neutralizing chemical.
Low-pH water can be treated via injection systems.
Maintenance
The chemical feed pump must be serviced and the chemical storage tanks refilled as with all chemical feed systems. Soda ash, rather than sodium hydroxide, is the favored chemical since it is safer to handle. Periodically inspecting and cleaning screens and filters is recommended.
Special considerations
When working with sodium hydroxide, exercise caution. To avoid inhaling vapors when adding it manually, make sure there is adequate ventilation. Take your time adding the chemical to the water and make sure it’s thoroughly mixed in. Wear protective gloves, goggles, and clothing to keep the chemical out of your eyes and on your skin. Cool, dry, and combustible items should be kept out of the reach of sodium hydroxide.
Before implementing an injectable system, people on a low sodium diet should speak with their doctor. Manufacturer requirements can be used to compare sodium levels in a water treatment system’s water to the sodium levels in the diet. It is possible to swap potassium hydroxide for sodium hydroxide, although it will cost more.
Two key aspects to know about your acid choice
Depending on their chemical formulae, acids can be categorised or grouped together in many ways, which can be helpful when deciding which acid to purchase and use. These classifications help us understand how acids interact with water and shed light on their propensity to really acidify agricultural drinking water.
In order to comprehend acid’s impact on water’s pH, it’s necessary to know at least one of two connected values: 1) Ka and 2) pKa for each acid.
Ka is the acid disassociation constant; to put it simply, Ka is the acid’s strength when it is dissolved in water. The acid gets stronger as the number increases. Because Ka values are commonly stated in scientific notation, it can be difficult to simply compare acids based on their Ka values. PKA values are used in this context.
It’s easier to represent the same value in the form of pKa, which is shown in the equation below. the stronger an acid is if its pKa value is negative.
In other words, pKa = -log (Ka)
Example:
Sulfuric acid is (H2SO4)
1.0 × 10-3 or.001
In other words, pKa = -log (1.0×10-3) =
(Using the pKa values makes it easy to compare the strengths of different acids.)
Strong acids versus weak acids
In solution, strong acids tend to dissociate into a proton H+ and an anion A-, whereas weak acids tend to remain in one form. Acids are classified as strong if their Ka and pKa values are more than or equal to 1, respectively (recall the relationship above). The pKa value of strong acids is greater than 1, while the ka values of weak acids are less than 1.
When strong acids dissolve fully in water, the equilibrium between strong and weak acids is maintained. How far this equilibrium may be reached is determined by the acid’s Ka and pKa levels and the water’s pH. A pH equivalent to the pKa value of the weak acid will result in a 50% HA (related) to 50% H+ + A- equilibrium (disassociated).
Acid dissociation at a high level:
In other words, HA equals H+ plus A-
(Acid breaks down fully into a proton and an ion.)
Xem thêm : The Perfect Type Of Chair For Tailbone Pain. Comprehensive Review
When it is put to water, anion
Dissociation of weak acids:
In other words, HA equals H+ plus A-.
(When acid is added to water, both the acid molecule and the disassociated proton and anion are in equilibrium.)
Monprotic acids (one H+) against polyprotic acids (more than one H+)
To lower the pH of poultry or swine drinking water, it is necessary to increase the concentration of hydrogen ions in the water. As a result, the H+ proton is the most important part of the acid molecule. An acid that can only donate one hydrogen ion to water is considered monoprotic (a single hydrogen H+). Acids can also be classed according to how many potential hydrogen ions they can contribute to water. Polyprotic acids are those that can donate more than one hydrogen ion to water (two or more free hydrogen protons). The number of hydrogen atoms a polyprotic acid can contribute helps to further categorize it.
A good example of a diprotic acid is sulfuric acid, which has the chemical formula H2SO4. Because of this, it is capable of donating up to two hydrogen ions to the solution.
As a triprotic (H3A – three hydrogen) acid, phosphoric acid has the ability to transfer up to three hydrogen ions to other acids. This does not guarantee, however, that an acid will always give all of its hydrogen ions to water because it contains many hydrogen atoms in its structure. As a result of the addition of polyprotic acids to water, specific Ka and pKa values are allocated to each dissociation process. These values rise with each succeeding disassociation, and the disassociations increase as a result.
Become less favorable (or, more difficult to use more of the H+ in that acid molecule). Sulfuric acid and phosphoric acid are also examples of this notion.
Examples:
Anhydrous Sulfuric Acid (H2SO4)
pKA = -3 for the first dissociation: HO2SO4 > H+ + HSO4
(very corrosive)
HSO4- H+ + SO4 – pKA = 1.92 in the second dissociation.
(slight acidity)
The pKa value of -3 for sulfuric acid indicates that it is a strong acid. Hydrogen sulfate’s pka value is larger than 1 after the initial disassociation, hence it is actually a weak acid in nature. The pH of the water has a direct effect on the amount of the second disassociation. (The second dissociation will be nearly complete for the purposes of farm animal drinking water.) Sulfuric acid is an excellent candidate for acidification in water with a naturally low pH because of this relationship.
Hexaprotic phosphoric acid: H3PO4.
Phosphoric acid dissociation in water is studied (this is when the relationship of pKa and pH and how it affects disassociation comes into play).
pKa = 2.12 first dissociation: H3PO4 H+ + H2PO4
(slight acidity)
After that, H2P04– H+ +HPO4-2 pKa = 7.21 is observed.
(slight acidity)
pKa = 12.31 for HP04 2- H+ + PO4 3- HP04 2-
(slight acidity)
While phosphoric acid can transfer three hydrogen atoms to water as a triprotic acid, its ability to do so depends on the pH of the farm drinking water to which it is added, because it is a weak acid. Phosphoric acid is a relatively poor acidifier in the field due to the link between pH and pka. Phosphoric acid’s second dissociation pKa value is 7.21. The equilibrium between H2PO4 and HPO4-2 is 50/50 at a pH of 7.21, as can be seen from this. Further pH lowering will be difficult unless substantial volumes of acid are provided to replace hydrogen protons generated from the first dissociation, as the available hydrogen protons can only be donated by another 50%.
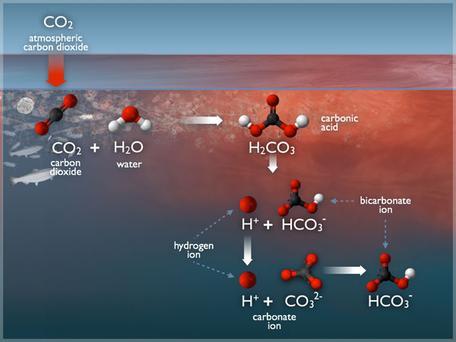
Organic acids versus inorganic acids (mineral acids)
The presence of carbon in the chemical makeup of organic and inorganic acids is the primary difference between them. Because this organic acid designation is entirely technical and does not apply to certified organic production of poultry or swine for slaughter, organic acids are not (necessarily) authorized any certification like OMRI. A carbon-based organic acid is distinct from a carbon-based inorganic acid. This is something to keep in mind as we progress through the rest of this article. In the field, inorganic acids will be the most common source of strong acids, whereas organic mixes containing weak acids will be the most common.
Using acids on the farm
You must know how farm water affects product efficacy when using acid on the farm, regardless of whether the acid is being used to acidify water for better water quality or to increase the solubility of water-soluble products (such as antibiotics). Also, how the water system’s health and the health of the birds could be impacted by the addition of these goods.
When using acidifiers, it is important to consider the chemical makeup of water coming from the farm’s wells. Considering the alkalinity and hardness of farm water is essential before adding acidifiers. The level of difficulty or ease with which the pH of the water can be altered will be directly related to the concentration of these analytes in the water.
A product that works well on one farm could not have substantial effects on another because of water chemistry differences (i.e., low vs high alkalinity) in the area. Due to enhanced buffering capacity—or “cancelling out” of the acid—the pH of water becomes more difficult to shift when alkalinity and hardness are higher.
The amount of acidifier that needs to be applied to reach the desired pH range can be readily and rapidly measured using affordable pH test strips or a water pH probe. In order to get the most out of your acidifier, you need take into account both the native farm well water’s chemical makeup and the acidifier’s features.
Acid injection
How acid injection works
It is possible to improve chlorination efficiency and get rid of the soda taste in water that has a high pH by using acid injection. As water with a pH above 9 can corrode brass, copper, zinc, aluminum, and iron, this approach also minimizes the risk of pipe corrosion.
A point of entry is a mechanism for injecting acid. Acetic acid (white vinegar) is injected into high pH water using a chemical feed pump composed of corrosion-resistant materials. Citric acid and alum can also be used, but they are more expensive. Alternatively, they can be substituted. In order to lower the pH, hydrochloric acid and sulfuric acid can be used, but they are more hazardous and require specific handling. However, if the pH of the untreated water is 11 or higher, they are suggested. In order to get tap water to a pH of around 7, add the acid solution and adjust the flow rate.
Maintenance
Xem thêm : How To Avoid Bedhead? Effective Guide For You!
Pump maintenance and chemical replenishment are required for all injection systems. When handling acid, wear goggles, gloves, and protective clothes.
Special considerations
Children should be kept away from acid injection system chemicals, which must be handled with care and stored in clearly marked containers. Acid solutions should always be diluted with water, not the other way around. Before purchasing an acid injection system or other chemicals, be sure to read the manufacturer’s instructions carefully.
Final considerations
The water system and the birds’ performance can be adversely affected if an unidentified product is added, and this knowledge is crucial whenever a product is put to water that will be consumed by the birds. The inclusion of carbon in the acid’s chemical composition distinguishes organic acids from inorganic ones, as discussed earlier in the piece. Carbon can be used as a food source by any microorganisms that may be residing inside the entire water system, despite the fact that this change appears insignificant at first (i.e., pressure regulators, pressure tanks, stand pipes, drinker lines). Birds’ water supply could be shut off by a “bio-bloom” on the inside of the waterlines if this prospective food source and pH shift produce the ideal conditions. Some of you may already be familiar with this on your own farms.
For this reason, organic acids should be used in conjunction with a biocidal molecule, such as hypochlorous acid (tablet), acid-activated chlorine dioxide or an EPA recognized biocidal peroxide, to prevent the formation of a bloom of organic acids in the water. When employing organic acids/blends to either alter pH or offer ideal GI tract acidification for bird gut health, it is vital that water line disinfectants be used between each flock.
Consider the acid’s composition and the anion(s) [A] remaining in the water, as well as their potential impact on birds, as the final piece of the puzzle. In order to achieve the appropriate pH range in highly buffered water, a sulfuric acid-based product and a considerable volume of acid may be used. This method, while uncommon, has the potential to significantly raise the concentration of sulfate ions in the water. As sulfates and magnesium combine, birds may have a laxative effect and a decrease in performance. Many other potential dangers and interactions must be considered when acidifiers or any other substance is added to farm water.
Water treatment isn’t difficult, but you must know and understand your farm’s drinking water chemistry before you can get solid advise from a respected technical supplier who isn’t just selling you a “one-size-fits-all” silver bullet. Please contact your MWI Animal Health Territory Manager if you have any issues about acids or water acidification. If you have any questions, please don’t hesitate to contact us.
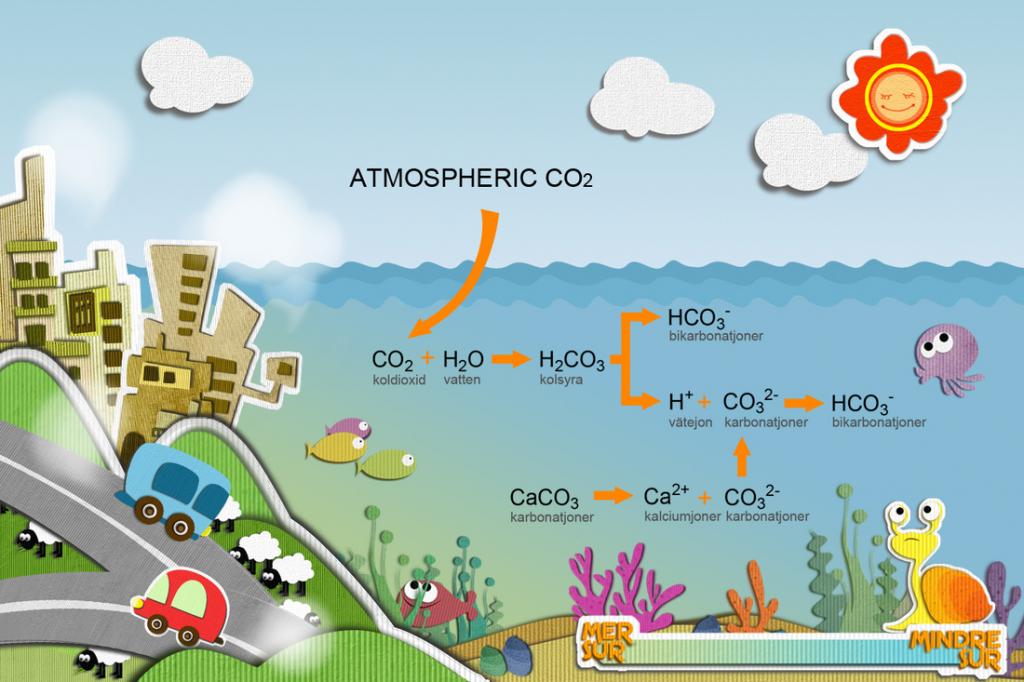
Tips on How to Acidify Water for Your Plants
Tip #1: Learn everything you need to know about pH levels
The acidity or alkalinity of a solution can be determined by measuring its pH value. Between 0 and 14 is the typical pH range. If the pH of the solution falls within the range of 0–7, then it is acidic.
As long as it’s between 7 and 14, it’s simple. Neutrality is defined as a pH value of 7.
Soda and vinegar are both acidic since they have a pH value of 3. To put it another way, soap has a pH value of 8. You need to know the ideal pH level for your plants in order to correctly acidify their water.
Water that is somewhat acidic is preferred by plants. This necessitates a pH range of between 5.0 and 6.5 in the water. However, many plant specialists regard a pH level of 5.5 to be neutral because it happens frequently in nature.
Tip #2: Know the right amount of acidity that is beneficial for your plants
The breakdown of organic matter in the ground is greatly facilitated by the presence of acidity. It also has an effect on the removal from the soil of harmful microorganisms, pesticides, heavy metals, and other food additives.
Your plants can be harmed if your water’s pH level is too high or too low. So, it’s critical that you know just how much acidity your plants need and get it just right.
However, how do you know if you’re heading in the proper direction or veering off course? There are a few things you should be aware of in order to tell if your water is overly acidic or basic.
High pH levels in plants can be detected, for example, by their leaves turning yellow-white and poor soil quality and soil life. Weak stems and discolored leaves are all symptoms of low pH in plants. Wilting leaves and stunted growth are all signs of high pH.
Tip #3: Consider the pH value of the growing medium around your plants
Hydroponically or not, the pH of the media in which your plants are growing is a crucial consideration. Plants thrive when the pH level is between 5.5 and 6.5.
Any deviation from this can have a substantial impact on their progress. It’s possible to resolve a problem with the pH level of your growth tool if you notice it.
Make use of nutrients and fertilizers that you are already familiar with. You must wait an hour before moving on to the next step for plants cultivated hydroponically. Wait 24 hours if you’re growing in dirt.
Use a pH test strip or a computerized test kit to check the pH of your growing equipment. You should read the instructions on the back of the kit before you begin.
Check your plant’s pH level using a test strip and determine if it’s within acceptable range. This will assist you determine whether or not you need to adjust the pH of your growth gear.
Fill a gallon of clean water to the appropriate level for the pH-altering substance you intend to use. To guarantee that the water-to-substance ratio is accurate, follow the instructions to the letter. A different volume of water may be required.
In order to adjust water’s pH, mix in wood ash, lime, or any other specially formulated solution. Phosphoric acid or sulfur can also be added to reduce the pH.
It’s important that the solid material in your combination is soaked before being added to the mixture. Adding it to the mixture will allow it to become infused with it. Use the combination on your plants as a fertilizer.
Find out if you got the pH level correct. If you didn’t, you may have to make a new batch until you get it right. Prior to testing hydroponically grown plants, you should wait 30 minutes and 24 hours before evaluating soil-grown plants.
Why Should You Grow Your Plants in a Mini Greenhouse?
Insects and other creatures like caterpillars can wreak havoc on your garden plants. Blights can also spread to other plants in your garden. Protect your plants at all times.
A greenhouse can be a cost-effective option for growing your plants. Putting up a greenhouse may seem like an impractical and costly endeavor.
However, greenhouses exist in various sizes. As long as it’s not too huge, it’s fine. In terms of size, you just need as much as you need it.
You can construct a small greenhouse in your yard if you’re short on room. Smaller greenhouses still have the same advantages as larger greenhouses, but they’re less expensive to run. Even if you have a patio or a balcony, you can use it.
Growing plants in a tiny greenhouse is a terrific way to get a head start on the cold season. Get your healthy plants into the ground as soon as it is safe to do so.
It can also be used to grow delicate perennials. During the winter, the little greenhouse will keep them safe from the snow and ice.
Final Thoughts on How to Acidify Water
Your plants will thank you if you know how to acidify their water. It can speed up the growth of your plants! First, conduct a pH test on your solution to determine the appropriate treatment for achieving the desired level of acidity.
Questions to ask before you buy
Get your water analyzed by a state-licensed laboratory before you buy a water purification system. Determine if pH modification is a suitable treatment strategy for your case by conducting this experiment There are several questions to ask before purchasing a water purification system.
Published with permission of Wagenet,L. ; Mancl K. ; Sailus M. (1995). Northeast Regional Agricultural Engineering Service, Cooperative Extension, NRAES-48, Ithaca, New York. Residential Water Treatment.
Nguồn: https://iatsabbioneta.org
Danh mục: Health